On April 30, 1897, British physicist and Nobel Laureate in Physics, Sir Joseph John Thomson (commonly known as J. J. Thompson, 18 December 1856 – 30 August 1940) announced the existence of electrons.
Thompson called the particles “corpuscles”, meaning “small bodies”, but later scientific community preferred the name electron which had been suggested by the Irish physicist George Johnstone Stoney in 1891 because these particles are the fundamental unit of electricity (see notes 1).
The word electron is a combination of the words electric and ion (a suffix, appearing in words of Latin origin, denoting action or condition). The suffix -on which is now used to designate other subatomic particles, such as a proton or neutron, is in turn derived from electron.
Today’s (April 30) story of what happened this day in Science, Technology, Astronomy, and Space Exploration history.
Discovery of the Electron
Despite the fact that J. J. Thomson was the director of the Cavendish Laboratory at the University of Cambridge and one of the most respected scientists in Great Britain, the scientific community found the existence of electrons hard to believe.
Scientists were thinking the atom was the smallest and indivisible part of matter that could exist. Because the atomic model proposed by the English chemist and physicist John Dalton (6 September 1766 – 27 July 1844) was prevalent. Dalton’s model suggested that the atom was the smallest and indivisible part of matter that could exist (like tiny, solid billiard balls).
Thomson’s discovery disproved Dalton’s model.
Cathode ray tube experiment
Thomson used a device called a cathode ray tube. It’s a big tube of glass, like a bottle. There’s no air in it, it’s a vacuum tube. The English chemist and physicist Sir William Crookes (17 June 1832 – 4 April 1919) developed the first cathode-ray tube in the 1870s.

There were two pieces of metal at the narrower end. Thomson connected these two pieces of metal to a DC power source and created a ray. This ray created a bright spot at the other end of the tube when it hit a special coating on the inside of the glass.
Then, he took two metal plates and inserted them on either side of the cathode ray tube (see the diagram below), and connected another DC power source to these plates. The top plate was positively charged while the bottom plate was negatively charged.
When he turned on the 1st power supply, again a ray was generated, but this is time, instead of going straight, it bent upwards, towards the positively charged plate.
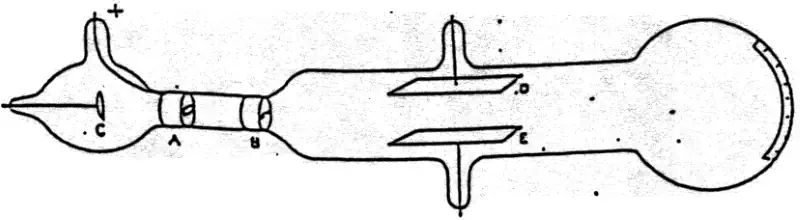
When the upper plate was connected to the negative pole of the battery and the lower plate to the positive pole, the glowing patch moved downwards, and when the polarity was reversed, the patch moved upwards.
The obvious outcome here is that the ray is made of stuff that is negatively charged.
J. J. Thomson combined the information that he got from his previous experiments, he concluded that the particles that made up cathode rays were a thousand times smaller than a hydrogen atom.
So, the experiments showed that the atoms had tiny, negatively charged particles inside them.
As explained above, Thomson called these tiny particles “corpuscles”, meaning “small bodies”, but later scientific community preferred the name electron which had been suggested by the Irish physicist George Johnstone Stoney in 1891 because these particles are the fundamental unit of electricity (see notes 1).
J.J. Thompson discovered the electron, the first of the subatomic particles, using the cathode ray tube experiment. He found that many different metals release cathode rays and that cathode rays were made of electrons, very small negatively charged particles. This disproved John Dalton’s theory of the atom (The first part of Dalton’s theory states that all matter is made of atoms, which are indivisible. The second part of the theory says all atoms of a given element are identical in mass and properties), and Thompson came up with the plum pudding model of the atom.
J. J. Thomson said:
“As the cathode rays carry a charge of negative electricity, are deflected by an electrostatic force as if they were negatively electrified, and are acted on by a magnetic force in just the way in which this force would act on a negatively electrified body moving along the path of these rays, I can see no escape from the conclusion that they are charges of negative electricity carried by particles of matter.”
As to the source of these particles, Thomson thought that they emerged from the molecules of gas in the vicinity of the cathode. He explained:
“If, in the very intense electric field in the neighborhood of the cathode, the molecules of the gas are dissociated and are split up, not into the ordinary chemical atoms, but into these primordial atoms, which we shall for brevity call corpuscles; and if these corpuscles are charged with electricity and projected from the cathode by the electric field, they would behave exactly like the cathode rays.”
Thomson imagined the atom as being made up of these corpuscles orbiting in a sea of positive charge; this was his plum pudding model.
This “plum pudding” model was later proved incorrect when his student Ernest Rutherford (30 August 1871 – 19 October 1937) showed that the positive charge is concentrated in the nucleus of the atom.
Rutherford described the atom as a tiny, very dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negatively charged electrons, circulate at some distance, much like planets revolving around the Sun.
Notes
- George Johnstone Stoney FRS (15 February 1826 – 5 July 1911) initially coined the term electrolion in 1881. Ten years later, he switched to electron to describe these elementary charges, writing in 1894: “… an estimate was made of the actual amount of this most remarkable fundamental unit of electricity, for which I have since ventured to suggest the name electron“.
Sources
- J. J. Thomson on Wikipedia
- Electron on Wikipedia
- John Dalton on Wikipedia
- “Dalton’s atomic theory” on the Khan Academy website
- Moon Landings: All-Time List [1966-2025] - February 2, 2025
- What Is Max-Q and Why Is It Important During Rocket Launches? - January 16, 2025
- Top 10 Tallest Rockets Ever Launched [2025 Update] - January 16, 2025