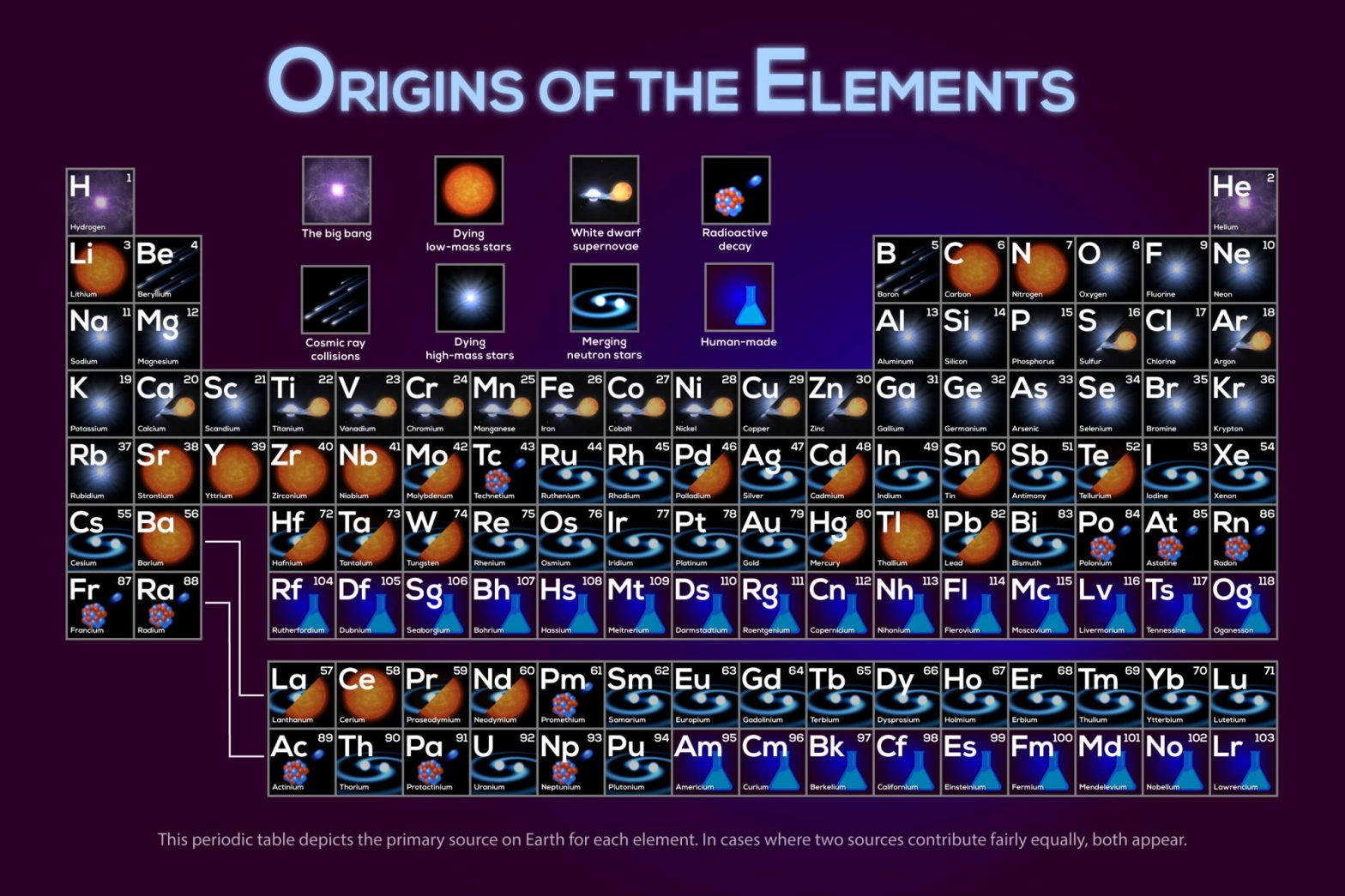
NASA Exoplanets has published an amazing infographic showing the origins of the elements. From the calcium in our bones to the iron in our blood, we really are made from star stuff.

Origins of the elements, by their atomic number
- Hydrogen (H): The Big Bang
- Helium (He): The Big Bang
- Lithium (Li): Dying low-mass stars (While lithium is created in stellar fusion synthesis, trace amounts of it were created in the big bang along with hydrogen, helium, and deuterium – source)
- Beryllium (Be): Cosmic ray collisions
- Boron (B): Cosmic ray collisions
- Carbon (C): Dying low-mass stars. Carbon is the basis for life as we know it.
- Nitrogen (N): Dying low-mass stars
- Oxygen (O): Dying high-mass stars
- Fluorine (F): Dying high-mass stars
- Neon (Ne): Dying high-mass stars
- Sodium (Na): Dying high-mass stars
- Magnesium (Mg): Dying high-mass stars
- Aluminum (Al): Dying high-mass stars
- Silicon (Si): Dying high-mass stars
- Phosphorus (P): Dying high-mass stars
- Sulfur (S): Dying high-mass stars / white dwarf supernovae
- Chlorine (Cl): Dying high-mass stars
- Argon (Ar): Dying high-mass stars / white dwarf supernovae
- Potassium (K): Dying high-mass stars
- Calcium (Ca): Dying high-mass stars / white dwarf supernovae
- Scandium (Sc): Dying high-mass stars
- Titanium (Ti): White dwarf supernovae
- Vanadium (V): White dwarf supernovae
- Chromium (Cr): White dwarf supernovae
- Manganese (Mn): White dwarf supernovae
- Iron (Fe): White dwarf supernovae
- Cobalt (Co): White dwarf supernovae
- Nickel (Ni): White dwarf supernovae
- Copper (Cu): Dying high-mass stars / white dwarf supernovae
- Zinc (Zn): Dying high-mass stars / white dwarf supernovae
- Gallium (Ga): Dying high-mass stars
- Germanium (Ge): Dying high-mass stars
- Arsenic (As): Dying high-mass stars
- Selenium (Se): Dying high-mass stars
- Bromine (Br): Dying high-mass stars
- Krypton (Kr): Dying high-mass stars
- Rubidium (Rb): Dying high-mass stars
- Strontium (Sr): Dying low-mass stars
- Yttrium (Y): Dying low-mass stars
- Zirconium (Zr): Dying low-mass stars
- Niobium (Nb): Dying low-mass stars
- Molybdenum (Mb): Merging neutron stars / Dying low-mass stars
- Technetium (Tc): Radioactive decay. It is the process by which an unstable atomic nucleus loses energy through radiation.
- Ruthenium (Ru): Merging neutron stars
- Rhodium (Rh): Merging neutron stars
- Palladium (Pd): Merging neutron stars / Dying low-mass stars
- Silver (Ag): Merging neutron stars
- Cadmium (Cd): Merging neutron stars / Dying low-mass stars
- Indium (In): Merging neutron stars
- Tin (Sn): Merging neutron stars / Dying low-mass stars
- Antimony (Sb): Merging neutron stars
- Tellurium (Te): Merging neutron stars / Dying low-mass stars
- Iodine (I): Merging neutron stars
- Xenon (Xe): Merging neutron stars
- Cesium (Cs): Merging neutron stars
- Barium (Ba): Dying low-mass stars
- Lanthanum (La): Merging neutron stars / Dying low-mass stars
- Cerium (Ce): Dying low-mass stars
- Praseodymium (Pr): Merging neutron stars / Dying low-mass stars
- Neodymium (Nd): Merging neutron stars / Dying low-mass stars
- Promethium (Pm): Radioactive decay
- Samarium (Sm): Merging neutron stars
- Europium (Eu): Merging neutron stars
- Gadolinium (Gd): Merging neutron stars
- Terbium (Tb): Merging neutron stars
- Dysprosium (Dy): Merging neutron stars
- Holmium (Ho): Merging neutron stars
- Erbium (Er): Merging neutron stars
- Thulium (Tm): Merging neutron stars
- Ytterbium (Yb): Merging neutron stars
- Lutetium (Lu): Merging neutron stars
- Hafnium (Hf): Merging neutron stars / Dying low-mass stars
- Tantalum (Ta): Merging neutron stars / Dying low-mass stars
- Tungsten (W): Merging neutron stars / Dying low-mass stars
- Rhenium (Re): Merging neutron stars
- Osmium (Os): Merging neutron stars
- Iridium (Ir): Merging neutron stars
- Platinum (Pt): Merging neutron stars
- Gold (Au): Merging neutron stars
- Mercury (Hg): Merging neutron stars / Dying low-mass stars
- Thallium (Tl): Dying low-mass stars
- Lead (Pb): Merging neutron stars / Dying low-mass stars
- Bismuth (Bi): Merging neutron stars
- Polonium (Po): Radioactive decay
- Astatine (At): Radioactive decay
- Radon (Rn): Radioactive decay
- Francium (Fr): Radioactive decay
- Radium (Ra): Radioactive decay
- Actinium (Ac): Radioactive decay
- Thorium (Th): Merging neutron stars
- Protactinium (Pa): Radioactive decay
- Uranium (U): Merging neutron stars
- Neptunium (Np): Radioactive decay
- Plutonium (Pu): Merging neutron stars
- Americium (Am): Human-made (first produced in 1944 as part of the Manhattan project)
- Curium (Cm): Human-made (First produced in 1944, using the cyclotron at Berkeley)
- Berkelium (Bk): Human-made (named after the city of Berkeley, California, the location of the Lawrence Berkeley National Laboratory where it was discovered in 1949)
- : Californium (Cf): Human-made (first synthesized in 1950 at Lawrence Berkeley National Laboratory)
- Einsteinium (Es): Human-made (named in honor of Albert Einstein, it was discovered as a component of the debris of the first hydrogen bomb explosion in 1952)
- Fermium (Fm): Human-made (discovered in the debris of the first hydrogen bomb explosion in 1952, and named after Enrico Fermi, one of the pioneers of nuclear physics)
- Mendelevium (Md): Human-made (discovered in 1955 by bombarding einsteinium with alpha particles. It was named after Dmitri Mendeleev, 1834-1907, father of the periodic table of the chemical elements.)
- Nobelium (No): Human-made (named in honor of Alfred Nobel)
- Lawrencium (Lr): Human-made (named in honor of Ernest Lawrence, 1901-1958, inventor of the cyclotron, a device that was used to discover many artificial radioactive elements)
- Rutherfordium (Rf): Human-made (named after Ernest Rutherford, first produced in the 1960s)
- Dubnium (Db): Human-made (first produced in 1968)
- Seaborgium (Sg): Human-made (named after the American nuclear chemist Glenn T. Seaborg, first produced in 1978)
- Bohrium (Bh): Human-made (First discovered in 1981, named to honor the Danish physicist Niels Bohr)
- Hassium (Hs): Human-made (Discovered in 1984)
- Meitnerium (Mt): Human-made (First produced in 1982, named after Lise Meitner, 1878-1968, a leading Austrian-Swedish physicist who was one of those responsible for the discovery of the element protactinium and nuclear fission)
- Darmstadtium (Ds): Human-made (first created in 1994 by the GSI Helmholtz Centre for Heavy Ion Research in the city of Darmstadt, Germany, after which it was named)
- Roentgenium (Rg): Human-made (first created in 1994 by the GSI Helmholtz Centre for Heavy Ion Research near Darmstadt, Germany. It is named after the physicist Wilhelm Röntgen, who discovered X-rays)
- Ununbium (Uub): Human-made (One atom of this element was detected on February 9, 1996, at the German Heavy-Ion Research Center (GSI) in Darmstadt. The atom was made by bombarding a lead target with high-energy zinc atoms.)
- Nihonium (Nh): Human-made (first created in 2003 by a Russian–American collaboration at the Joint Institute for Nuclear Research -JINR- in Dubna, Russia)
- Flerovium (Fl): Human-made (named after Russian physicist Georgy Flyorov, 1913-1990, discovered in 1998 at the Flerov Laboratory of Nuclear Reactions of the Joint Institute for Nuclear Research in Dubna, Russia)
- Moscovium (Mv): Human-made (first synthesized in 2003 by a joint team of Russian and American scientists at the Joint Institute for Nuclear Research (JINR) in Dubna, Russia)
- Livermorium (Lv): Human-made (named after the Lawrence Livermore National Laboratory in the United States, which collaborated with the Joint Institute for Nuclear Research -JINR- in Dubna, Russia to discover livermorium during experiments conducted between 2000 and 2006)
- Tennessine (Ts): Human-made (announced in Dubna, Russia, by a Russian-American collaboration in April 2010. As of 2022, it is the most recently discovered element)
- Oganesson (Og): Human-made (first synthesized in 2002 at the Joint Institute for Nuclear Research -JINR- in Dubna, near Moscow, Russia. The name honors the Russian-Armenian nuclear physicist Yuri Oganessian, born 14 April 1933, who played a leading role in the discovery of the heaviest elements in the periodic table.)
The origins of the elements
- The hydrogen in our bodies, present in every molecule of water (H2O), came from the Big Bang. There are no other appreciable sources of hydrogen in the universe.
- The carbon in our bodies was made by nuclear fusion in the interior of stars, as was oxygen. Stars shine by burning hydrogen into helium in their cores, and later in their lives create heavier elements. Most stars have small amounts of heavier elements like carbon, nitrogen, oxygen, and iron, which were created by stars that existed before them. After a star runs out of fuel, it ejects much of its material back into space going novae or supernovae. The major difference between a nova and a supernova is that in a supernova a lot of the object’s mass is ejected with the explosion (and some of this ejected material creates new stars and planets, like our sun and the solar system planets, and the elements like carbon and oxygen in our bodies come from this ejected material). The amount of this mass is more than the mass of the sun. Whereas in a nova, very less mass is ejected as compared to that in a supernova. A nova also does not destroy its host star whereas a supernova does.
- White dwarf supernova: in this type of supernova, a white dwarf in a binary star system gains mass from its companion. As its mass increases, the temperature and pressure within the white dwarf also increase, until nuclear fusion re-ignites at its center. A white dwarf is so dense that fusion sweeps rapidly out from the center, releasing a tremendous amount of energy that blows the star apart.
- Much of the iron in your body was made during supernovas of stars that occurred long ago and far away.
- The gold in your jewelry was likely made from neutron star mergers that may have been visible as short-duration gamma-ray bursts or gravitational wave events.

Related: Stardust is Raining Down on Earth
Carl Sagan – Cosmos: Stars – We Are Their Children
The surface of the Earth is the shore of the cosmic ocean. On this shore, we’ve learned most of what we know. Recently, we’ve waded a little way out, maybe ankle-deep, and the water seems inviting. Some part of our being knows this is where we came from. We long to return, and we can, because the cosmos is also within us. We’re made of star stuff. We are a way for the cosmos to know itself.
Carl Sagan
Sources
- “The Origins of the Elements” on the NASA Science website
- “Differences Between a Nova and a Supernova” on the Difference Between website
- “White Dwarf Supernova” on the American Museum of Natural History website
- How Many Elephants are Left in the World in 2025? - August 17, 2025
- Moon Landings: All-Time List [1966-2025] - February 2, 2025
- What Is Max-Q and Why Is It Important During Rocket Launches? - January 16, 2025